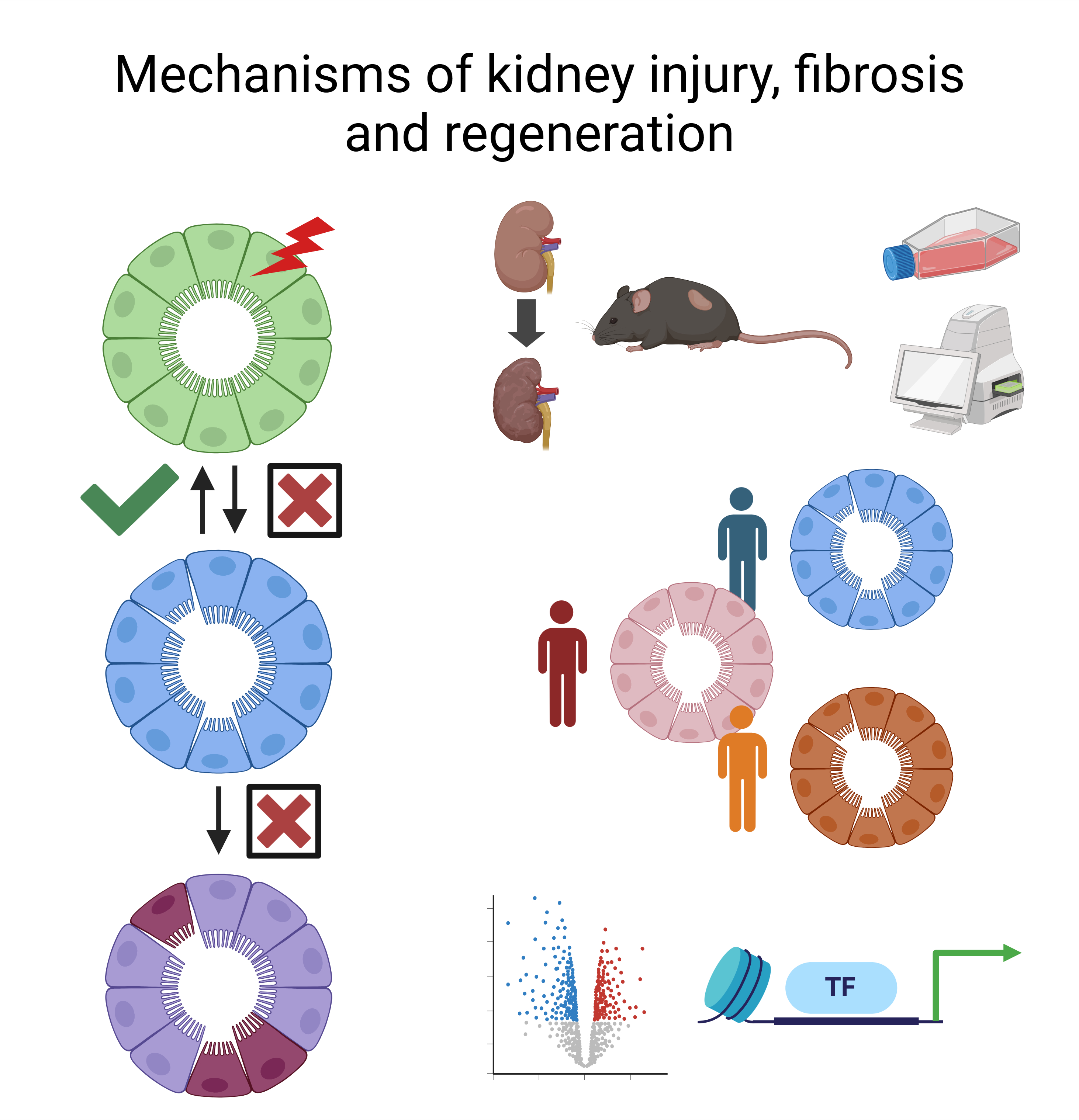
Kidney injury and fibrosis
Kidney fibrosis represents the final common pathway of all chronic kidney diseases (CKD). Key challenges in addressing kidney fibrosis and halting CKD progression include understanding how to effectively target the health-to-disease transition of tubular epithelial cells and discovering strategies to promote cell redifferentiation and regeneration following kidney injury.
Our research integrates human clinical resources, mouse models of kidney injury and fibrosis, tissue culture systems, real-time metabolic measurements, multiomics and bioinformatics approaches to uncover the genetic and epigenetic mechanisms driving kidney diseases.
Kidney fibrosis represents the final common pathway of all chronic kidney diseases (CKD). Key challenges in addressing kidney fibrosis and halting CKD progression include understanding how to effectively target the health-to-disease transition of tubular epithelial cells and discovering strategies to promote cell redifferentiation and regeneration following kidney injury.
Our research integrates human clinical resources, mouse models of kidney injury and fibrosis, tissue culture systems, real-time metabolic measurements, multiomics and bioinformatics approaches to uncover the genetic and epigenetic mechanisms driving kidney diseases.
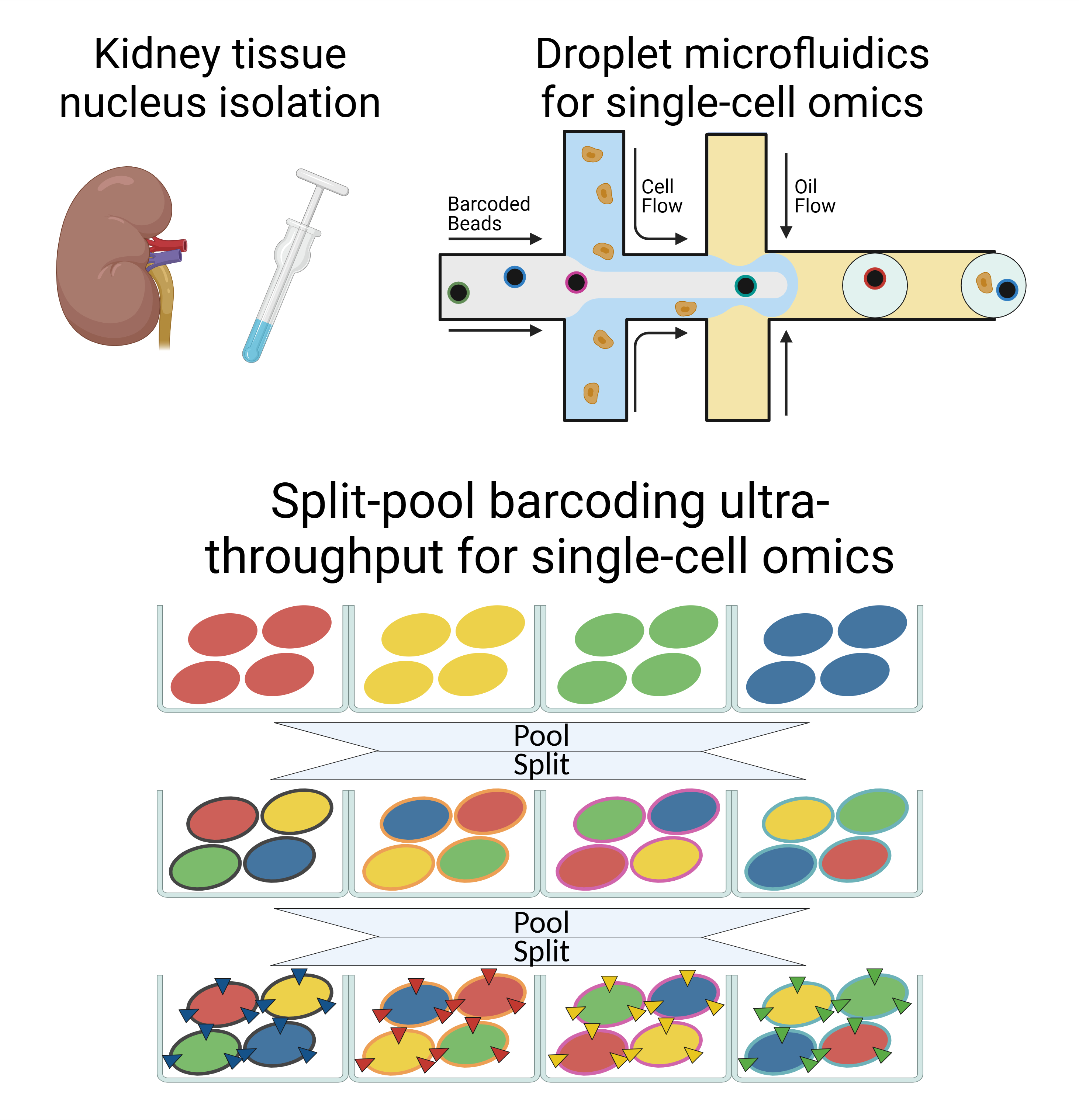
Human diseases at the single-cell resolution
We have deep expertise in development and applications of various single-cell technologies. Beyond the well-known droplet microfluidics approach, we employ the split-pool barcoding method, which offers unique advantages in ultra-high throughput, robust sample multiplexing and cost efficiency.
The creation of the kidney single-cell transcriptomics reference atlas (Human Cell Atlas v1) marked a significant milestone. Now, we stand at the forefront of a transformative era, poised to develop a more comprehensive atlas — one characterized by greater coverage, a broader spectrum of disease subtypes, and enhanced multimodal complexity (HCA v2).
We have deep expertise in development and applications of various single-cell technologies. Beyond the well-known droplet microfluidics approach, we employ the split-pool barcoding method, which offers unique advantages in ultra-high throughput, robust sample multiplexing and cost efficiency.
The creation of the kidney single-cell transcriptomics reference atlas (Human Cell Atlas v1) marked a significant milestone. Now, we stand at the forefront of a transformative era, poised to develop a more comprehensive atlas — one characterized by greater coverage, a broader spectrum of disease subtypes, and enhanced multimodal complexity (HCA v2).
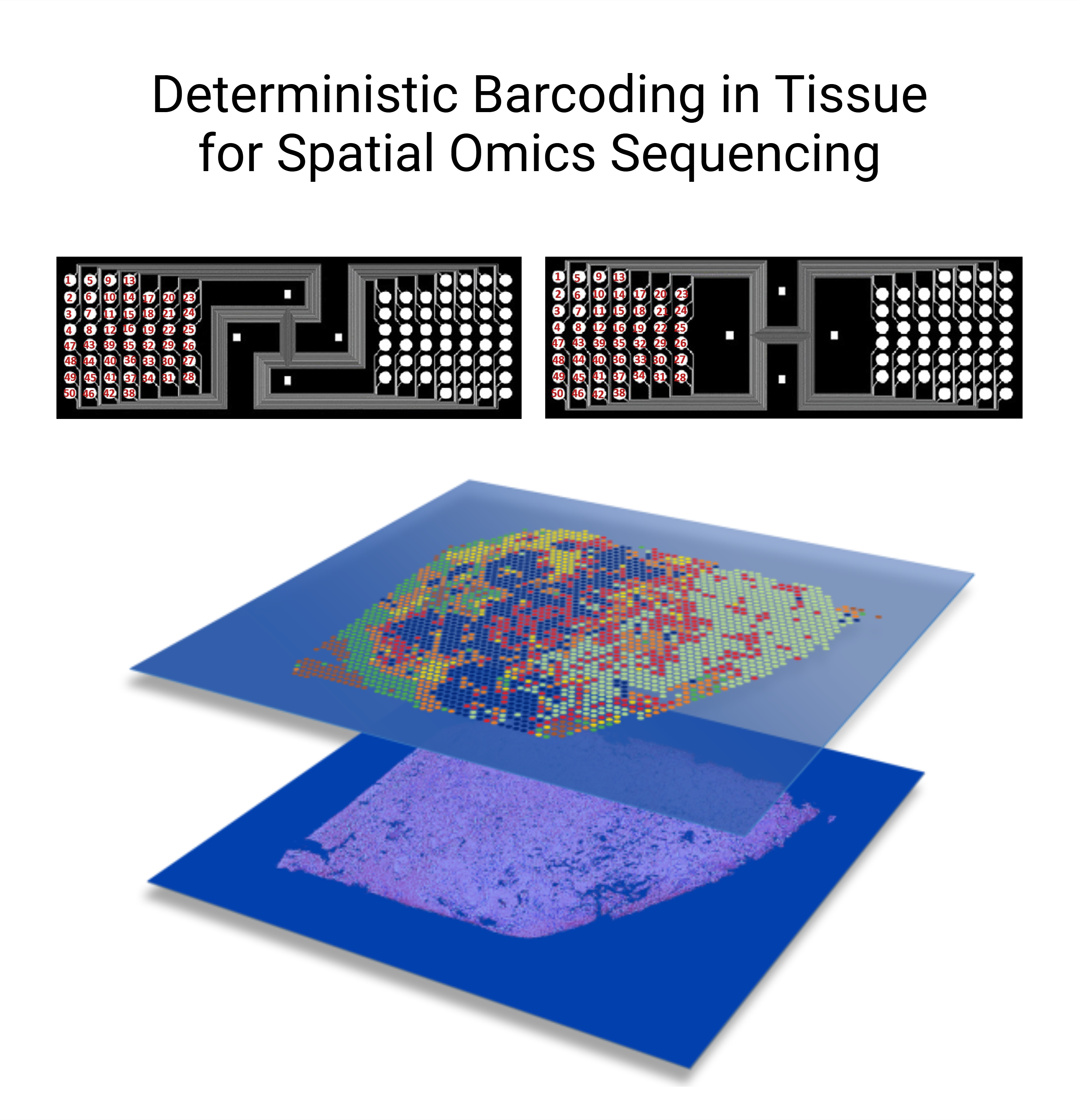
Spatially resolved multiomics
Never before have we had the ability to observe a tissue section at such a comprehensive molecular level and high resolution as we do today. The advent of spatial multiomics offers us invaluable opportunities to deepen our understanding of the structural changes in tissue organization and alterations in cell communication mechanisms underlying kidney physiology and disease states.
We are using cutting-edge Deterministic Barcoding in Tissue (DBiT) approaches to achieve near-single-cell resolution and whole-genome coverage for exploring spatial transcriptomics and spatial epigenomics. Furthermore, we aim to develop methods to enable spatial multimodal profiling and integrative analysis.
Never before have we had the ability to observe a tissue section at such a comprehensive molecular level and high resolution as we do today. The advent of spatial multiomics offers us invaluable opportunities to deepen our understanding of the structural changes in tissue organization and alterations in cell communication mechanisms underlying kidney physiology and disease states.
We are using cutting-edge Deterministic Barcoding in Tissue (DBiT) approaches to achieve near-single-cell resolution and whole-genome coverage for exploring spatial transcriptomics and spatial epigenomics. Furthermore, we aim to develop methods to enable spatial multimodal profiling and integrative analysis.